
Calderón, Jaramillo, Ríos, Brito
MONITORING OF
MICROBIAL
POPULATION OF
THE
PÁRAMO SOIL OF THE CHARGE ZONE OF
LAKE
MAPAHUIÑA
IN
CHIMBORAZO-ECUADOR
Cristina Calderón
1*
, David Jaramillo
1
, Anita Ríos
1
, Guido Brito
2
Universidad Nacional de Chimborazo (UNACH)
1
, Av. Antonio José de Sucre
Km
11
/ vía a Guano, Riobamba, Ecuador.
*
email: cristy.gct@gmail.com
2
Escuela Superior Politécnica de Chimborazo (ESPOCH)
2
, Panamericana Sur km
11/2, Riobamba, Ecuador.
R
esumen
A
bstract
Peru and Bolivia, covering
the Andes region with
Introduction
altitudes ranging from 3000 to over 4800 meters above
sea level (1). Given its hydro-physical properties, it has
a high capacity to retain water due to the andosol soils
of volcanic origin with a presence of peat and a dense
herbaceous vegetation cover (2). Due to the altitude at
which it is located, the temperature decreases and there is
a presence of fog (3). Being in the Equatorial region, the
The páramo is an ecosystem composed
of grasslands combined with small
remaining vestiges of native forest and
in recent years an increased presence of
foreign plantations. In South America
it extends from Costa Rica to northern
23
The present investigation analyzed the presence of microorganisms in soils of the recharge zone of
the Lake Mapahuiña, Ecuador. The location has a large microbial diversity which is characterized
by a páramo ecosystem presenting acidic soil types, which coincides with other paramunos volcanic
soils analyzed in the region. The general microbial analysis revealed a considerable amount of
microorganisms in each region of study, with no significant correlation found regarding the physical
and chemical characteristics measured. For microalgae, the banks of the Lake Mapahuiña had the
highest concentration (5,5 x 10
5
cells/mm
3
) in contrast to the Pine forest area (1,7 x 10
5
cells/mm
3
).
Microalgae growth showed significant differences with respect to pH, relative soil humidity, and
vegetation type with soil temperature relatively homogeneous. Our study is the first investigation
of the area and presents the foundation for unearthing microbial strains of biotechnological interest.
Keywords: soil, páramo, bacterial, microalgae, microbial count
La presente investigación analiza la presencia de microorganismos en los suelos de la zona de
recarga de la Laguna Mapahuiña, Ecuador. Cuenta con gran diversidad microbiana que caracteriza
un ecosistema páramo con presencia de suelos ácidos, como otros suelos volcánicos paramunos
analizados en la región. El análisis microbiano general reveló una considerable cantidad de
microorganismos en la región de estudio, no se encontró correlación significativa respecto a las
características físicas y químicas obtenidas. La concentración más alta (5,5 x 10
5
células / mm
3
) de
microalgas se presentó a orillas de la laguna Mapahuiña, en contraste con el área de bosque de pino
(1,7 x 10
5
células / mm
3
). El crecimiento de microalgas mostró diferencias significativas con respecto
al pH, humedad relativa del suelo, y el tipo de vegetación con temperatura del suelo relativamente
homogénea. Nuestro estudio es la primera investigación de la zona y presenta las bases para revelar
cepas microbianas de interés biotecnológico.
Palabras claves: suelo, páramo, bacteriana, microalgas, recuento microbiano
climate is stable throughout the year, although there is a
marked difference between day and night, with overnight
temperatures dropping significantly. Yet the páramo
shows a great diversity of living systems such as plants,
birds, amphibians, mammals and a microbiological
component (4,5).
Materials and Methods
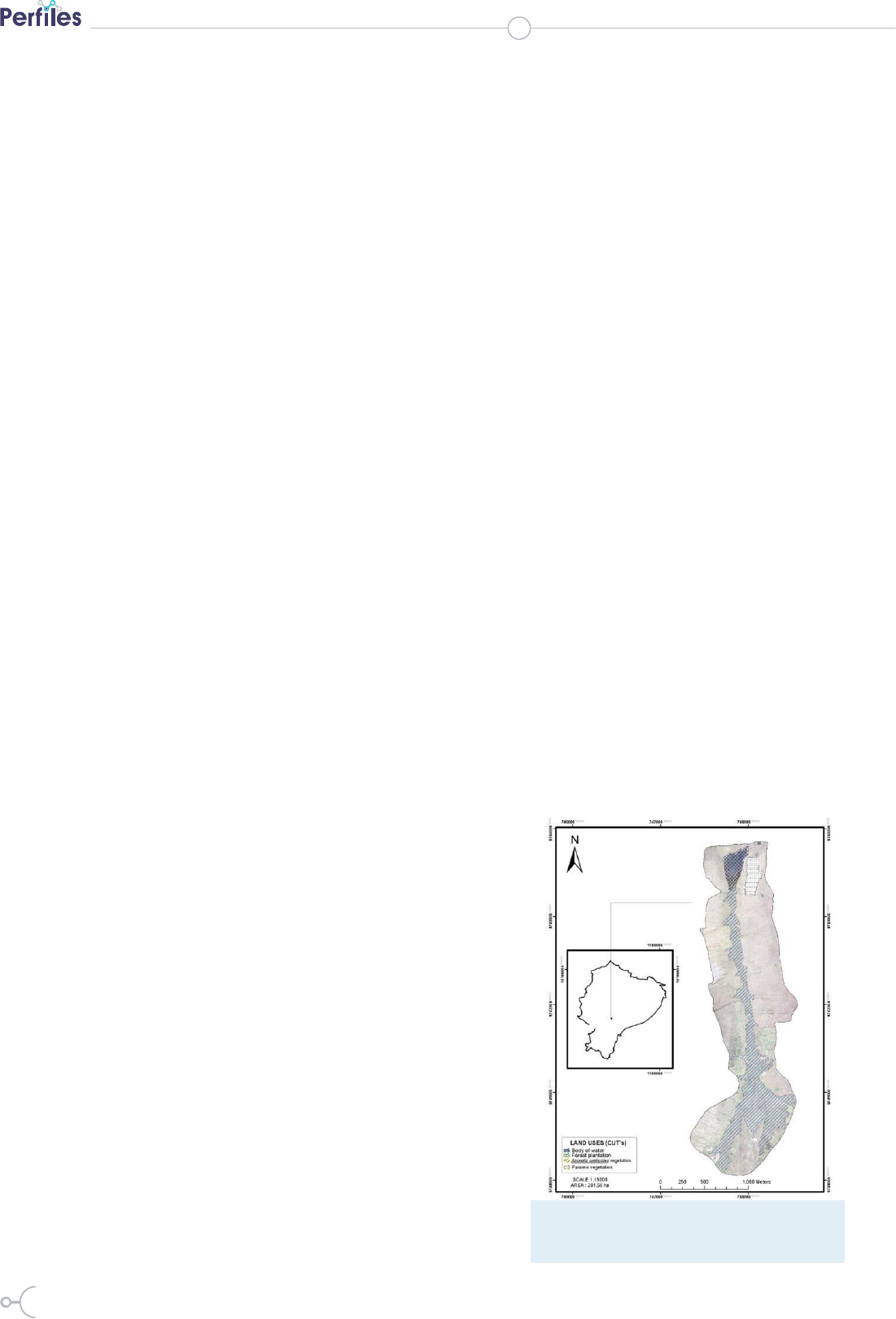
Area of study
This study was conducted in the
recharge zone of Lake Mapahuiña
(9742946 N, 747817 E) in the Sangay
National Park (Chimborazo Province),
which belongs to the microbasin of
the Zula River, having an oval-oblong
morphometry of 281,542 ha of mainly
sandy loam soil. The average daily
temperature is between 6-12
o
C, with a
daytime maximum of 15
o
C and a night-
time minimum of 3
o
C recorded.
Despite the topographical variability, andosols of the
páramo are defined by a remarkable homogeneity in
their physicochemical properties (6,7). The degradation
and changes in land use threaten the hydrology of the
páramo by influencing the cycles of carbon, nitrogen,
sulfur and phosphorus (2,8) , which are implicated in the
activity of soil microorganisms and soil mineralization
processes (9,10). Among the colonizing páramo soil
microorganisms are the microalgae (11), which generally
grow with low insolation and are exposed to extreme
seasonal fluctuations in temperature UV radiation and
desiccation.
The annual rainfall of the zone is
between 700-1000 mm . The life zones in
its páramo grassland ecosystem include
lower montane dry and wet forests which
contribute to its vast ecological diversity
(20, 21). The samples were collected and
analyzed weekly between September
and December 2013, months in which
the weather conditions were suitable to
attend the study site. Figure 1 outlines
the various sampling areas according
to land use which include: Region 1:
páramo vegetation, with Culcitium
canescens Humb. & Bonpl, tristerix
longebracteatus and the endemic grass
Stipa ichu; Region 2: Azorella aretioides
They also serve as potential bio-indicators of the degree
of conservation of the ecosystem (12,13). In addition
to its role in regulating soil properties, microalgae are
also the source of biomolecules and metabolites of great
economic importance used primarily in food, medicines,
fertilizers and biofuels (14–16) motivating further
scientific
efforts to discover new microorganisms, as in the
newly identified cyanobacteria found in the páramo zone
of Costa Rica (17). Algal activity depends on the internal
cycles and partnerships between these microorganisms,
interactions with organic and inorganic nutrients derived
from animal or vegetable debris and exposure to surface
runoff (18). Furthermore, páramo soil microalgae are
capable of creating symbiotic associations with other
soil microorganisms like bacteria, promoting the growth
of plants through the production of the auxin indole-3-
acetic acid (19).
In Ecuador, the páramo is located in the highlands of
the Andes occupying an area of approximately 12,650
km2, about 5% of the territory. According to Mena (4),
Ecuador is the country with the largest amount of páramo
with respect to its land extension, yet little is known about
its edaphic fauna and even less of its vast microalgae
wealth. This research aims to conduct monitoring of the
population of soil microorganisms, with special emphasis
on microalgae, by determining whether physicochemical
soil variables such as pH, moisture and temperature
influence their distribution and concentration.
24
Figure 1: Map of study area: Region 1, páramo
vegetation; Region 2, Azorella aretioides vegetation;
Region 3, Pinus radiata forest and Region 4, Contours
of lake Mapahuiña
ISSN 1390-5740 Número 15 Vol. 1 (2016)
ISSN 2477-9105
Calderón, Jaramillo, Ríos, Brito
vegetation, incorporating Stipa ichu,
Lachemilla orbiculata and the grass
Calamagrostis intermedia; Region 3:
Pinus radiata forest and Region 4: the
contours of Lake Mapahuiña, composed
of volcanic rocks and Azorella aretioides
(21).
Mapahuiña
†All permanent monitoring plots distributed every 50 meters unless stated; Region
1: monitoring plots distributed to account for runoff areas and convergence zones;
Region 2: sample plots excluded waterlogged areas; Region 3: number of plots based
on the region’s area %; Estimation of plot number used a sampling error of 10%
(Schlegel et al., 2011)
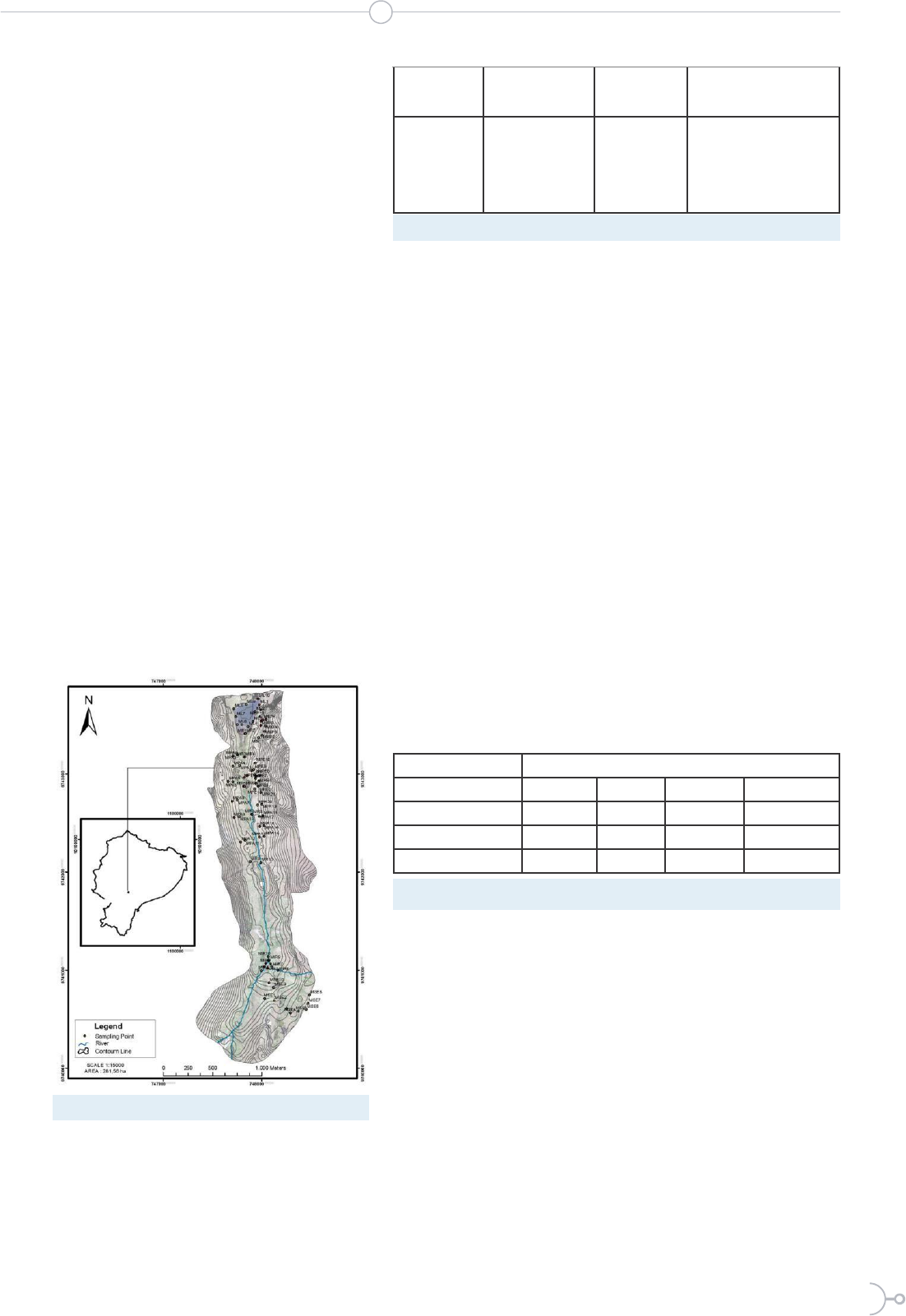
Soil sampling
The sampling scheme was based on
the surface density of land use (20)
together with the aid of base mapping,
Soil analysis
accessibility
and
satellite
imagery,
placing 86 monitoring points in the
recharge zone of Lake Mapahuiña
(Figure 2, Table 1). The soil sampling
included taking a portion of soil (200 g,
depth: ~20 cm) using a plot (1 x 1 m) from
the study area, which had an average
altitude of 4130 meters above sea level.
Sample integrity was maintained with
the use of resealable plastic bags and
Subsamples of surface sediment (10 g) were diluted in
peptone water to achieve a stock concentration of 10
-10
as
previously described. Microalgal quantification was de-
termined as cells/mm
3
(24) by direct microscopic counts.
For the quantification of heterotrophic bacteria, a diluted
sample (1 mL, 10
-6
) was added to a Petrifilm
TM
aerobic
bacteria plate (3M, USA) and incubated for 3 days at
26
o
C. Similarly, yeast and mould colonies (1 mL, 10
-3
)
were inoculated on PetrifilmTM yeast and mould type
plates, respectively (3M, USA) and incubated for 5 days
at room temperature. Microscopic colony enumeration
was determined as the number of colony forming units
(CFU) per gram of dry soil (CFU/g dry soil) (26).
cold storage during transportation
to the laboratory for analysis.
(22)
†Data collection: relative humidity measured with a soil hygrometer (I.C.T.S.L.,
Spain); pH measured with a portable pH meter (Hanna Instruments, USA);
temperature recorded with a digital ground thermometer (Hanna Instruments, USA).
Statistical Analysis
Data analysis was carried out using the software packages
ArcGIS Geostatistical Analyst (Esri, 10.1), which utilizes
Inverse Distance Weighing (IDW) for multivariate
interpolation, (27) and the statistical software InfoStat.
Differences between regions for each variable were
evaluated by ANOVA and Tukey
‟
s test, with the non-
parametric method of Kruskal–Wallis also employed.
Results
25
Figure 2: Sampling point distribution of the study area
Table 2: Physical and chemical characteristics of soil samples from the
recharge zone of Lake Mapahuiña
Region
Characteristic
†
1
2
3
4
Moisture (%)
15-80
35-90
30-40
30-85
Temperature (
o
C)
5.1-13.9
7.0-11.1
6.9-10.8
10.0-16.1
pH
4.5-6.4
4.6-6.1
5.1-6.6
5.4-7.1
Region
Surface
area (ha)
Area
(%)
Plots
implemented
†
1
2
3
4
205.36
62.01
5.84
7.62
72.94
22.03
2.07
2.71
47
24
5
10
Table 1: Amount of surface area per land use in the recharge zone of Lake
Results
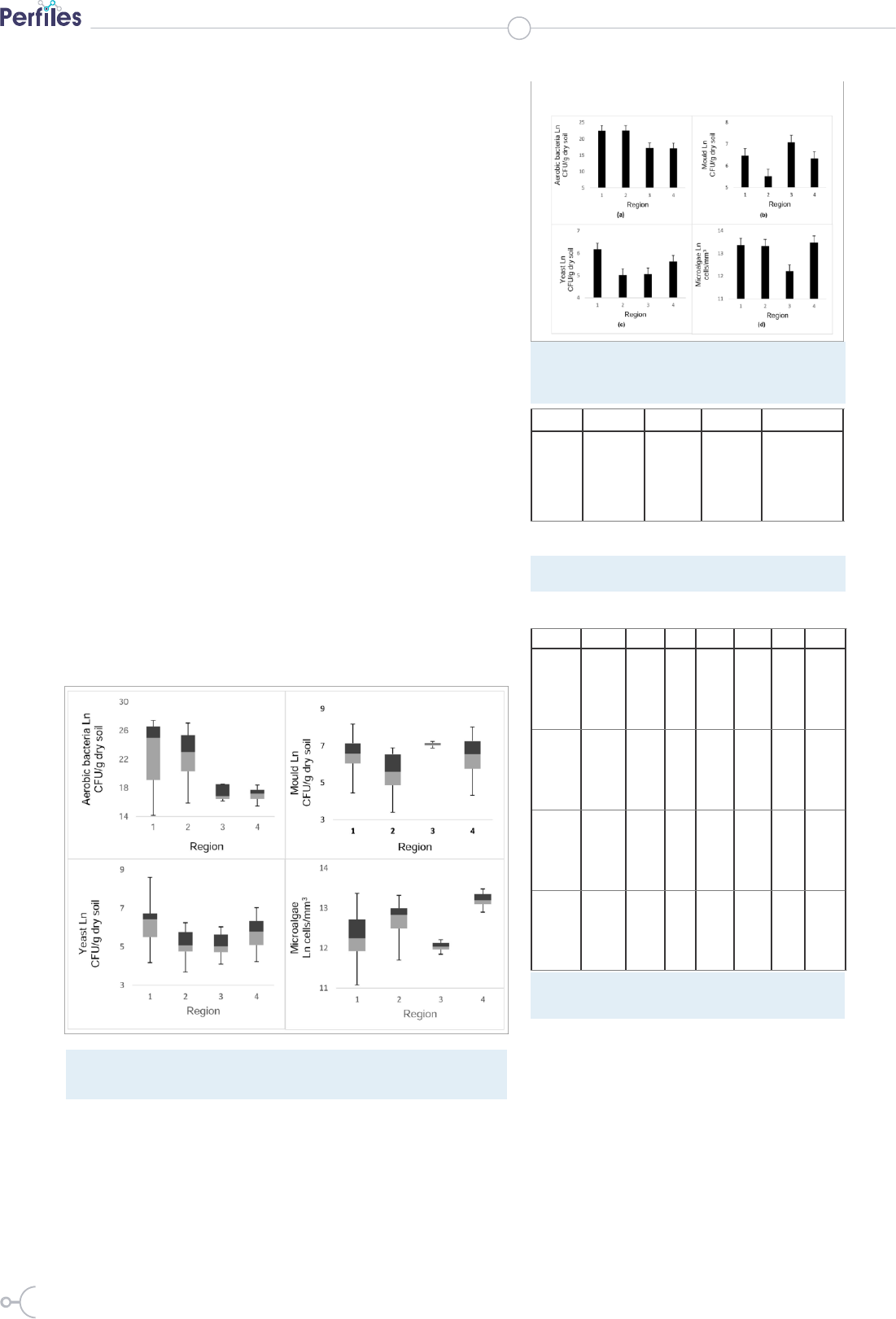
To describe the variation of the microbial abundance
present in each region of study (Table 3), descriptive
statistics for natural log (ln) of CFU/ g dry soil are shown
in Table 4 and illustrated graphically in Figures 3-4.
The influence of pH on the growth of bacteria was not
significant (Spearman r = -0.41; P <0.001), even with
adequate moisture (Spearman r = 0.24; P = 0.004) and
temperature (Spearman r = -0.09; P = 0.46). Similarly,
the amount of mould was affected by the pH of the soil
and showed a weak relationship (Spearman r = 0.06;
P = 0.60), however no correlation with temperature
(Spearman r = -0.15; P = 0.24) or moisture was observed
(Spearman r = -0.59; P <0.001). There was no significant
change in the amount of yeast observed within the soil
with changes in pH (Spearman r = -0.09; P = 0.48); a
weak correlation was obtained for both temperature
(Spearman r = -0.13; P = 0.27) and moisture (Spearman
r = -0.39; P <0.001). Similar to the other microorganisms
evaluated, the relationship between microalgae and soil
pH was not significant (Spearman r = 0.31, P <0.001)
while a weak correlation for temperature (Spearman r
= 0.45; P <0.001), and moisture (Spearman r = 0.41; P
<0.001) was observed.
Table 3: Number of microorganisms determined for
each study region†
†Data generated by statistical software InfoStat;
ln = natural logarithm; N = samples; Min. = mini-
mum value recorded; Max. = maximum value re-
corded; SD = standard deviation; Var = variance
Discussion
Bacteria
The study was performed in a specific
location amid different land uses where
distinctive factors are influencing the
soil bacterial communities. Throughout
26
Figure 3: Box plot of data for bacteria, mould, yeast (CFU/g dry soil) and
microalgae colonies (cells/mm3) established in the soil regions of the recharge
zone of Lake Mapahuiña
Region
ln
N
Min.
Max.
SD
Var
Bacteria
1
2
3
4
22.99
22.75
17.23
17.11
117
66
9
28
14.18
15.87
16.20
15.47
27.41
27.03
18.53
18.39
4.59
2.92
1.00
0.79
20.85
8.42
0.89
0.60
Mould
1
2
3
4
6.53
5.60
7.08
6.43
102
56
12
30
4.44
3.40
6.89
4.32
8.18
6.90
7.24
8.03
0.08
0.12
0.03
0.20
0.71
0.85
0.01
1.12
Yeast
1
2
3
4
6.26
5.08
6.00
5.68
110
47
24
30
4.17
3.69
4.09
4.22
8.61
6.25
7.35
7.04
1.04
0.70
1.03
0.80
1.08
0.48
1.02
0.62
Micro
algae
1
2
3
4
12.27
12.71
12.04
13.21
125
59
15
29
11.08
11.70
11.85
12.90
13.37
13.32
12.21
13.48
0.55
0.40
0.11
0.18
0.30
0.16
0.01
0.03
Table 4: Descriptive statistics† for microorganis-
ms (Ln) for the four study regions of the recharge
zone of Lake Mapahuiña
†All values in CFU/g of dry soil; ‡cells/mm
3
Figure 4: Number of microorganisms per soil region
of the recharge zone of Lake Mapahuiña: (a) aerobic
bacteria, (b) mould, (c) yeast and (d) microalgae
colonies.
Region
Bacteria
Mould
Yeast
Microalgae
‡
1
2
3
4
1.98 x 10
11
8.06 x 10
10
4.80 x 10
7
3.48 x 10
7
9.43 x 10
2
3.91 x 10
2
1.20 x 10
3
9.83 x 10
2
9.06 x 10
2
2.01 x 10
2
6.15 x 10
2
3.86 x 10
2
2.49 x 10
5
3.56 x 10
5
1.71 x 10
5
5.52 x 10
5
ISSN 1390-5740 Número 15 Vol. 1 (2016)
ISSN 2477-9105

Calderón, Jaramillo, Ríos, Brito
the recharge zone of Lake Mapahuiña
decrease the fungal biomass in plantations containing
this plant species (28).
Fungi more readily adapt to conditions of low soil
moisture than bacteria (34), with the ability of these
organisms to translocate water and support growth
in materials or sites where there is no adequate water
supply for growth. Region 1 showed a lower range in soil
moisture, compared with Region 4 and 2, which had the
following extent of moulds recorded.
The growths of bacteria and fungi in cold climates are
affected by the soil temperature range, with optimum
temperature growth below 30 °C and high temperatures
decreasing their activity (35). Although a weak correlation
was observed, there is not a wide range of soil temperature
variability, with the highest temperature value recorded
at 16 °C in Region 4 (Table 2). Accordingly, continual
monitoring of temperature fluctuations in the study area
may help to validate any alterations in bacteria and fungi
populations, indicative of disturbances in the region.
the bacterial
in Regions 1
abundance was highest
(páramo vegetation) and
2
(Azorella
aretioides
vegetation),
followed by Region 3 (Pinus radiata
forest) and lastly Region 4 (Contours
of Lake Mapahuiña) which produced
the smallest bacterial count recorded.
In particular, the availability of soil
nutrients
under
the
introduced
P.
radiata (Region 3) may be restricted by
polyphenolic compounds present in pine
conifers while the volcanic soil material
present in Region 4 has been shown to
reduce the respiration rate of soil bacteria
(28). With no
significant
correlation with
the physico-chemical characteristics of
the soil and temperature and humidity
generating a weak association towards
microbial growth, the bacterial diversity
and populations may be more dependent
on the elemental availability from
organic and inorganic matter (29,30)
present in the rich taxonomical diversity
of the plant communities in Regions 1
and 2. The distribution of bacteria in the
study site was not uniform (ANOVA
analysis), suggesting a connection to
land use, type of vegetation in the regions
(31) and several biotic factors including
soil organisms and abiotic changes in
nutrient supply of parent material (32).
Yeast
Yeasts were observed at lower abundance than bacteria
and are unevenly distributed both in number and species
type. In our study, the approximate yeast counts ranged
between 5.1 to 6.3 ln CFU/g dry soil (Table 4), which
is comparable to the study reported by Mestre (36) and
is in good agreement with previous determinations (37).
No significant correlation between pH and yeast growth
was observed, in accordance with the study of di Menna
(38), although Region 1 with a slightly more acidic soil
pH was determined to have the highest concentration of
yeast compared to the other regions.
Moulds
Microalgae
The
results
indicate significant
differences (ANOVA test; p <0.05)
in the distribution of mould in the soil
amid further evidence of the influence
of different land uses on their growth
as proposed by the study of Lauber
(33). As shown in Figure 3, Regions 1
(páramo vegetation) and 4 (Contours
of Lake Mapahuiña) were determined
to have the greatest concentration of
mould, followed by Region 2 (Azorella
aretioides vegetation) and the lowest
populations were observed in Region 3
(Pinus radiata forest). Here, the presence
of pine as previously concluded, can
As members of the soil microbial community, the impor-
tance of microalgae is its ability to contribute to the sta-
bility of the soil. A relationship between its concentration
and the physicochemical characteristics of the region was
not observed (Pearson r<0.5), concurring with previous
studies on the dependence of edaphic factors for the
propagation of microalgae (29,39). However the IDW
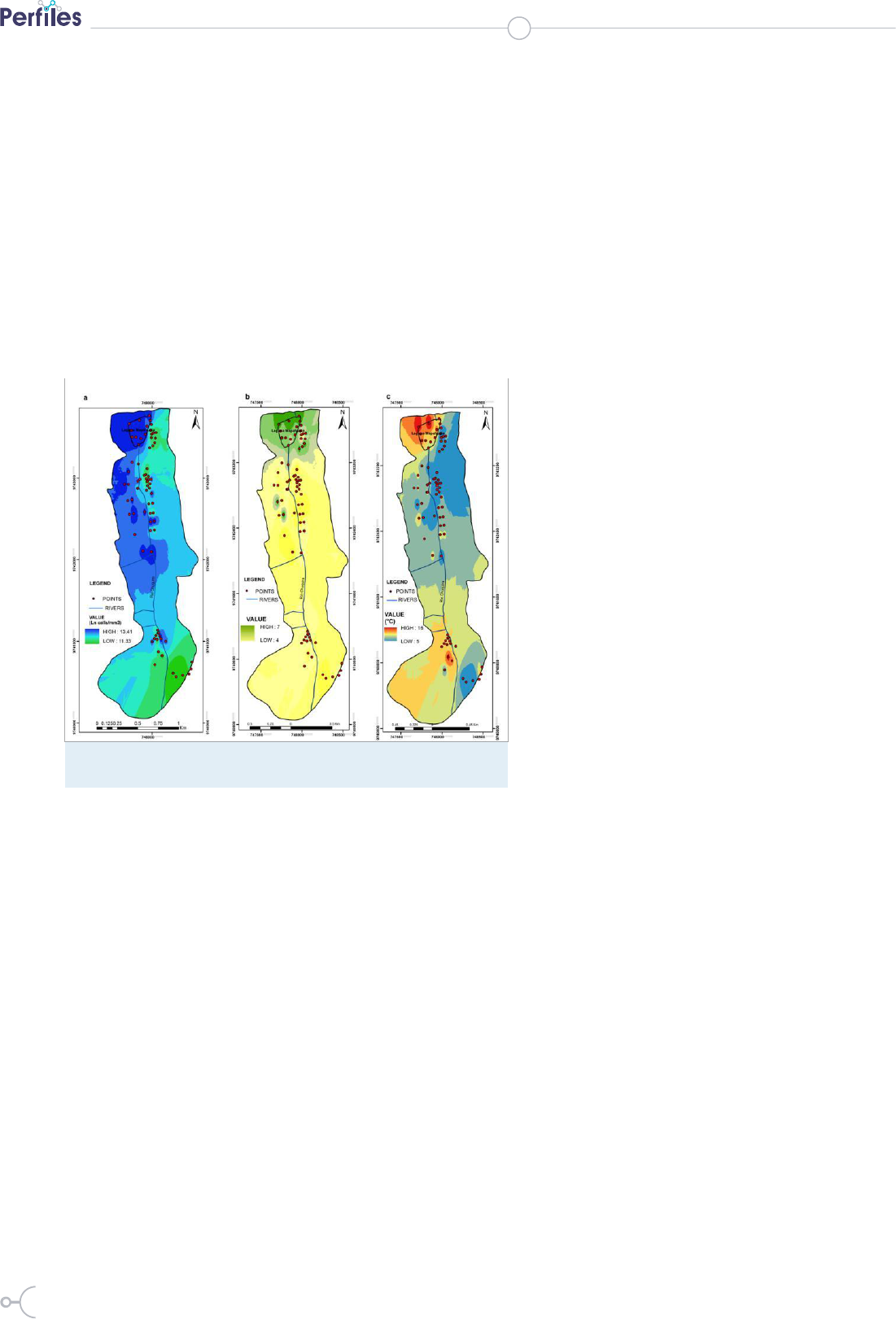
analysis (Figure 5) of Region 4 shows the highest dis-
tribution of microalgae, corroborating with the existence
of favorable conditions for microalgae development in
close proximity to aquifers and wetland areas (29,40).
Region 3 reported the smallest microalgae concentra-
tions and together with the lowest recorded soil moisture
content may be responsible for interrupting microalgae
27
cellular processes (24). It is postulated that the observed
differences in microalgae concentration and distribution
is related to the various processes of colonization in pá-
ramo ecosystems. The difference in variability appears
to be due to the study area and type of vegetation pre-
sent at each region. Nevertheless, it should be emphasi-
zed that in the studied area of the recharge zone of Lake
Mapahuiña in the Sangay National Park, a considerable
amount of microalgae was found, having the potential of
unearthing strains of interest for scientific and industrial
applications.
The recharge area of the microbasin
of the river Zula, which is part of the
Andean highlands, contains a significant
amount of microorganisms and remains a
favorable candidate to provide bacterial
strains with possible biotechnological
interest.
Acknowledgements
The authors would like to thank Patricio
Santillan and Franklin Cargua for their
help in base mapping; to Professor Janice
Aldrich-Wright from the University of
Western Sydney, for the revision of the
manuscript; to the Ecuadorian Ministry
of Environment (MAE) for generating
the necessary permits to conduct field
sampling in the buffer zone of the Sangay
National Park and to the residents of
the community of Mapahuiña for their
assistance with access to the study site.
Conclusions
The regions containing native páramo vegetation and
on the banks of Lake Mapahuiña, the greatest wealth of
microorganisms was recorded which was in contrast to
the plantation of Pinus radiata which possibly influences
the optimal growth of bacteria, microalgae and moulds.
Soil pH in general demonstrates a positive correlation
with bacterial growth; however, by having an acidic
medium the study site may contain acidobacterial
communities which counter the effect. The abundance
of microorganisms varies by land use and vegetation
type contained in each region. A direct dependence on
physicochemical factors such as pH, temperature and
humidity was not clear and further analysis of more
soil edaphic factors is needed to better understand the
microbial behavior. Microalgae were found throughout
the study area with a higher amount recorded in the
region with a body of water. Moisture at this site remains
a characteristic for its optimum growth.
28
Figure 5: Distribution of microalgae with respect to moisture (a), pH (b) and
temperature (c). Graphical analysis generated with ArcGIS Geostatistica
Analyst.
ISSN 1390-5740 Número 15 Vol. 1 (2016)
ISSN 2477-9105

Calderón, Jaramillo, Ríos, Brito
R
eferencias
1.
Ramsay P, Oxley E. Fire temperatures and postfire plant community dynamics in Ecuadorian
2.
Buytaert W, Wyseure G, De
Bièvre B,
Deckers
J.
The
effect
of land-use changes on the hydrologi-
5.
Hofman J,
Bezchlebová J,
Dusek
L, Dolezal L,
Holoubek I, Andel P, et al. Novel approach to
m
o
-
6.
Instituto Internacional de Agricultura Tropical (IITA), Fao. Manual de Prácticas integradas de
7.
Buytaert W, Deckers J, Wyseure G. Description and
classification
of nonallophanic Andosols in
9.
Ledin M. Accumulation of metals by microorganisms - processes and importance for soil sys-
10.
Zak
DR,
Pregitzer
KS, King JS,
Holmes WE.
RC05-Elevated
atmospheric CO2,
fine
roots and
t
h
e
14.
Johnston HW. The
Biological
and Economic Importance of
Algae.
Part 4: the Industrial
C
u
l
t
u-
15.
Hiroaki I. Industrial Production of
Microalgal Cell-mass
and
Secondary
Products- Major I
ndu
s
-
trial
Species
Chlorella. In: Richmond A, editor. Handbook of Microalgal Culture:
Biotechnology a
nd
16. Colorado M, Moreno D, Pérez
J.
Desarrollo , producción y
beneficio
ambiental de la p
r
o
d
ucció
n
de microalgas . La experiencia en La Guajira , Colombia
*
Development , Production and En
v
iro
n-
17.
Hauer T, Bohunická M, Mühlsteinová R. Calochaete gen. nov. (Cyanobacteria, Nostocales), a
18.
Ramírez M.
Revista
electrónica _Colombia tiene páramos_. La importancia de los microo
r
ga
-
19.
Gómez L, Valero N, De Brigard R. Halotolerant / alkalophilic bacteria associated with the cya-
nobacterium Arthrospira platensis ( Nordstedt ) Gomont that promote early growth in Sorghum
20.
Aguayo M, Pauchard A, Azócar G, Parra O. Cambio del uso del suelo en el centro sur de Chile
21.
Ministerio de Ambiente del Ecuador. Sistema de
clasificación
de los ecosistemas del Ecuador
22.
Oosporas D De, Phytophthora D, Montes GR, Fitosanidad I De, Posgraduados C De, Saldaña
HL, et al. del Valle de Toluca , México Phytophthora infestans Oospore Distribution in Soil of
t
h
e
29
1. Ramsay P, Oxley E. Fire temperatures and postfire plant community dynamics in Ecuadoria
grass paramo P. M.
Ramsay
1 & E. R.
B. Oxley.
Vegetatio. 1996;(124):129–173.
2. Buytaert W, Wyseure G, De
Bièvre B,
Deckers
J.
The
effect
of land-use changes on the hydrologi
cal behaviour of Histic Andosols in south Ecuador. Hydrol Process. 2005;19(20):3985–4082.
3.
Safford
HD.
Brazilian
Paramos I. An introduction to the
physical
environment and vegetation o
the campos de altitude. J
Biogeogr.
1999;26(4):693–712.
4. Mena, PV; Hofstede R.
Los
páramos ecuatorianos. Bot Econ los Andes Cent. 2006;91–109.
5. Hofman J,
Bezchlebová J,
Dusek
L, Dolezal L,
Holoubek I, Andel P, et al. Novel approach to
m
o
nitoring of the soil
biological
quality. Environ Int. 2003;28(8):771–779.
6. Instituto Internacional de Agricultura Tropical (IITA), Fao. Manual de Prácticas integradas d
manejo y conservación de
suelos.
Curso Capacit sobre el Manejo y Conserv
Suelos
IITA. 1997;1–238.
7. Buytaert W, Deckers J, Wyseure G. Description and
classification
of nonallophanic Andosols i
south Ecuadorian alpine grasslands (p??ramo). Geomorphology. 2006;73(3-4):207–228.
8. Zúñiga F. Técnicas de muestreo para manejadores de recursos naturales. 2004.
9. Ledin M. Accumulation of metals by microorganisms - processes and importance for soil sys
tems. Earth
Science Reviews.
2000;1–31.
10. Zak
DR,
Pregitzer
KS, King JS,
Holmes WE.
RC05-Elevated
atmospheric CO2,
fine
roots and
t
h
response of soil microorganisms: a
review
and hypothesis. New Phytol. 2000;147(1):201–223.
11. Mataloni G, Tell G, Wynn-Williams DD. Structure and diversity of soil
algal
communities
f
r
o
m
Cierva Point (Antarctic Peninsula).
2000;(15):205–116.
12. Lanza-Espino GD
La,
Hernandez-Pulido S, Carbajal-Pérez J. Organismos indicadores de la ca
lidad del agua y de la contaminación (bioindicadores).
2000.
p. 652.
13. Wynn-Williams DD. Response of pioneer soil microalgal colonists to environmental change in
Antarctica. Vol. 31, Microbial
Ecology.
1996.
14. Johnston HW. The
Biological
and Economic Importance of
Algae.
Part 4: the Industrial
C
u
l
t
u
ring of
Algae.
Vol. 22, Tuatara: Journal of the
Biological Society.
p. 1–164.
15. Hiroaki I. Industrial Production of
Microalgal Cell-mass
and
Secondary
Products- Major I
ndu
s
trial
Species
Chlorella. In: Richmond A, editor. Handbook of Microalgal Culture:
Biotechnology a
n
Applied
Phycology. Blackwell Science
Ltd;
2004.
p. 255–318.
16. Colorado M, Moreno D, Pérez
J.
Desarrollo , producción y
beneficio
ambiental de la p
r
o
d
ucció
de microalgas . La experiencia en La Guajira , Colombia
*
Development , Production and Environ
mental
Benefits.
2013;(5).
17. Hauer T, Bohunická M, Mühlsteinová R. Calochaete gen. nov. (Cyanobacteria, Nostocales),
new cyanobacterial type from the “páramo” zone in Costa
Rica.
Phytotaxa. 2013;109(1):36–44.
18. Ramírez M.
Revista
electrónica _Colombia tiene páramos_. La importancia de los microorga
nismos y la Edafofauna en los Páramos.
Bogotá;
2011 Feb;42–57.
19. Gómez L, Valero N, De Brigard R. Halotolerant / alkalophilic bacteria associated with the cya
nobacterium Arthrospira platensis ( Nordstedt ) Gomont that promote early growth in Sorghu
bicolor ( L .) Moench
Bacterias
halotolerantes /
alcalofilas
asociadas a la cianobacteria Arthrospira p.
2012;30(1):111–116.
20. Aguayo M, Pauchard A, Azócar G, Parra O. Cambio del uso del suelo en el centro sur de Chi
a fines del siglo XX . Entendiendo la dinámica espacial y temporal del paisaje. Rev Chil Hist Nat.
2009;(82):361–435.
21. Ministerio de Ambiente del Ecuador. Sistema de
clasificación
de los ecosistemas del Ecuado
continental. Subsecretaría de Patrimonio Natural (a). 2012;143.
22. Oosporas D De, Phytophthora D, Montes GR, Fitosanidad I De, Posgraduados C De, Saldañ
HL, et al. del Valle de Toluca , México Phytophthora infestans Oospore Distribution in Soil of
t
h
Toluca
Valley
,
Mexico.
2011;29:25–38.

26.
Crecchio C, Gelsomino A, Ambrosoli R, Minati JL, Ruggiero P. Functional and molecular
27.
Álvarez D, Matiz J, Cárdenas A. Modelos digitales batimétricos generados por métodos de
interpolación idw , kriging , Shepard y
B-Spline
en el archipiélago de Islas del Rosario Bathymetric
29.
Kastovská K, Elster J, Stibal M, Santrůcková
H.
Microbial assemblages
in
soil
microbial succession
characteristics on the diversity of bacteria in the southern brazilian atlantic forest. Appl Environ
33.
Lauber CL, Hamady M, Knight R, Fierer N. Pyrosequencing-based assessment of soil pH as a
35.
Pettersson M, Bååth
E.
Temperature-dependent changes in the
soil
bacterial community in lim
e
d
36.
Mestre MC, Libkind D, Fontenla
S.
Comparación de condiciones de cultivo para el aislamiento
39.
Davey MC, Rothery P. Primary colonization by microalgae in relation to spatial variation in
40.
Giraldo M. Aislamiento y caracterización de microalgas formadoras de tapetes microbianos
asociados a un cultivo hidropónico de plantas halófitas Isolation and Characterization of The
30
23. American
S
o
ciet
y
o
f
A
g
r
o
n
o
m
y.
Part
2.
En
A.
Wollum, Cultural Methods
f
o
r
S
o
i
l
Microorganisms.
In: Method of
Soil Analysis. 1982.
p. 785–792.
24. Díaz C, Molina X, Montecino V. Manual para el Monitoreo e Identificación de la Microalga
Bentónica Didymosphenia geminata. 2011;
25. 3M. Catalogo de producto_ 3M Seguridad de los Alimentos. 2013.
26. Crecchio C, Gelsomino A, Ambrosoli R, Minati JL, Ruggiero P. Functional and molecula
responses of soil microbial communities under differing soil management practices. Soil Bio
Biochem. 2004;36(11):1873–1956.
27. Álvarez D, Matiz J, Cárdenas A. Modelos digitales batimétricos generados por métodos
interpolación idw , kriging , Shepard y
B-Spline
en el archipiélago de Islas del Rosario Bathymetri
digital models generated by interpolation methods IDW ,
Kriging
, Shepard and
B-Spline
in the arch.
2011;3–14.
28. Iovieno P, Alfani A, Bååth E. Soil microbial community structure and biomass as affected by
Pinus pinea plantation in two Mediterranean areas. Appl
Soil Ecol. Elsevier B.V.;
2010;45(1):56–63.
29.
Kastovská K, Elster J, Stibal M, Santrůcková
H.
Microbial assemblages
in
soil
microbial successio
after
glacial
retreat in
Svalbard
(high arctic). Microb
Ecol.
2005;50(3):396–407.
30. Faoro H, Alves a. C, Souza EM, Rigo LU, Cruz LM, Al-Janabi SM, et al. Influence of soi
characteristics on the diversity of bacteria in the southern brazilian atlantic forest. Appl Enviro
Microbiol. 2010;76(14):4744–4753.
31. Garbeva P, van Veen
J
a, van
Elsas JD.
Microbial
diversity
in
soil:
selection microbial pop
u
l
a
t
io
n
by plant and soil type and implications for disease suppressiveness. Vol. 42, Annual review o
phytopathology.
2004.
p. 243–313.
32. Wardle D
a. The influence of
biotic interactions on
soil biodiversity. Ecol Lett.
2006;9(7):870–956.
33. Lauber CL, Hamady M, Knight R, Fierer N. Pyrosequencing-based assessment of soil pH as
predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol.
2009;75(15):5111–5131.
34. Jensen KD, Beier C, Michelsen A, Emmett BA.
Effects
of experimental drought on microbia
processes
in
two
temperate heathlands at contrasting
water
conditions.
Appl Soil Ecol.
2003;24(2):165–
246.
35. Pettersson M, Bååth
E.
Temperature-dependent changes in the
soil
bacterial community in lime
and unlimed soil.
FEMS
Microbiol
Ecol.
2003;45(1):13–21.
36. Mestre MC, Libkind D, Fontenla
S.
Comparación de condiciones de cultivo para el aislamient
y recuento simultáneo de levaduras de suelos de bosques nativos de Nothofagus spp. (FAGACEAE
de la Patagonia Argentina. Vol. 44. 2009.
37. Botha A. The importance and
ecology
of
yeasts
in soil.
Soil Biol
Biochem.
2011
Jan;43(1):1–8.
38. di Menna ME.
Yeasts
in Antarctic
soils.
Vol. 32, Antonie van
Leeuwenhoek. 1966.
p. 29–38.
39. Davey MC, Rothery P. Primary colonization by microalgae in relation to spatial variation i
edaphic factors on Antarctic
fellfield soils.
J
Ecol.
1993;81(2):335–378.
40. Giraldo M. Aislamiento y caracterización de microalgas formadoras de tapetes microb
ia
n
asociados a un cultivo hidropónico de plantas halófitas Isolation and Characterization of Th
Microbial Mats
Associated
to a Hydroponic Culture of Halophytic Plants.
ISSN 1390-5740 Número 15 Vol. 1 (2016)
ISSN 2477-9105